- Research
- Open access
- Published:
Discrepancy between cardiorespiratory system and skeletal muscle in elite cyclists after hypoxic training
Dynamic Medicine volume 2, Article number: 4 (2003)
Abstract
Background
The purpose of this study was to determine the effects of hypoxic training on the cardiorespiratory system and skeletal muscle among well-trained endurance athletes in a randomized cross-over design.
Methods
Eight junior national level competitive cyclists were separated into two groups; Group A trained under normoxic condition (21% O2) for 2 hours/day, 3 days/week for 3 weeks while Group B used the same training protocol under hypoxic condition (15% O2). After 3 weeks of each initial training condition, five weeks of self-training under usual field conditions intervened before the training condition was switched from NT to HT in Group A, from HT to NT in Group B. The subjects were tested at sea level before and after each training period. O2 uptake (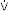 O2), blood samples, and muscle deoxygenation were measured during bicycle exercise test.
O2), blood samples, and muscle deoxygenation were measured during bicycle exercise test.
Results and Discussion
No changes in maximal workload, arterial O2 content, 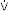 O2 at lactate threshold and
O2 at lactate threshold and 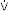 O2max were observed before or after each training period. In contrast, deoxygenation change during submaximal exercise in the vastus lateralis was significantly higher at HT than NT (p < 0.01). In addition, half time of oxygenation recovery was significantly faster after HT (13.2 ± 2.6 sec) than NT (18.8 ± 2.7 sec) (p < 0.001).
O2max were observed before or after each training period. In contrast, deoxygenation change during submaximal exercise in the vastus lateralis was significantly higher at HT than NT (p < 0.01). In addition, half time of oxygenation recovery was significantly faster after HT (13.2 ± 2.6 sec) than NT (18.8 ± 2.7 sec) (p < 0.001).
Conclusions
Three weeks of HT may not give an additional performance benefit at sea level for elite competitive cyclists, even though HT may induce some physiological adaptations on muscle tissue level.
Background
High altitude training (HAT) is used by many athletes for the purpose of enhancing performance. The scientific theory behind HAT is related to the fact that exposure to high altitude produces an increase in red blood cell (RBC) mass and hemoglobin (Hb) concentration, thereby enhancing the blood's oxygen transport capacity [1, 2]. In addition, HAT has been shown to increase skeletal muscle capillarization[3, 4]. A number of favorable physiological changes have also been observed within the skeletal muscle microstructure as a result of HAT. These changes include increased concentration of myoglobin (Mb) [5], increased mitochondrial oxidative enzyme activity [5], and a greater number of mitochondria [3].
In contrast, however, some studies have reported that endurance performance was not much improved by HAT compared with sea level training [6, 7]. Moreover, Levine and Stray-Gundersen insisted that HAT would lead to down regulation of muscle structure and function associated with reduced power output and reduced O2 flux [2]. The reasons for these divergent results may include the differences in exercise type, training intensity, training period and training altitude. Also, the subjects of HAT studies are usually divided into groups and then compared as to the effects of hypoxic training and normoxic training. Therefore, substantial interindividual variability in the adaptive response to HAT is involved in these studies [8]. To our knowledge, there is no formal study that reported the effects of HAT among identical subjects in athletes.
Recently, near infrared continuous wave spectroscopy (NIRcws) technology has been updated and widely applied for the evaluation of muscle tissue oxygenation [9, 10]. Muscle oxygenation as observed by NIRcws is considered to reflect the balance between O2 consumption and O2 supply, as demonstrated by its gradual decrease during incremental exercise, and by its dramatic increase after whole body exercise [9, 11, 12]. If the HAT improves muscle capillarization and/or mitochondrial oxidative capacity, muscle oxygenation during and after exercise should be modified by HAT. The purpose of this study was to determine the effects of hypoxic training on the cardiorespiratory system and skeletal muscle among well-trained endurance athletes in a randomized cross-over design.
Methods
Subjects
The subjects for this study were eight junior national level competitive young cyclists in Norway. The group included one female and seven male athletes (age: 17 ± 1 year, height: 180 ± 5 cm, weight: 70.6 ± 7.8 kg). Subjects gave their informed consent after obtaining all the information they requested concerning this study. To avoid acclimatization in hypoxic condition, the subjects lived at sea level and trained under normoxic and simulated hypoxic conditions. It is reported that some competitive athletes have a serum ferritin level that is suggestive of reduced bone marrow stores [17, 18]. When such athletes attempt hypoxic training, they often do not thrive, and clearly do not increase erythrocyte volume or 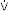 O2max. Therefore, all the subjects took iron supplement during the study. The subjects took liquid iron supplementation during the study.
O2max. Therefore, all the subjects took iron supplement during the study. The subjects took liquid iron supplementation during the study.
Training Protocol
The subjects were randomly assigned to two training groups. Group A (n = 4) trained under normoxic condition (NC; 21% O2) for 2 hours/day, 3 days/week for 3 weeks while Group B (n = 4) used the same training protocol under hypoxic condition (HC; 15% O2). After each training period, five weeks of self-training under usual field conditions was implemented as a washout period. After the self-training, training conditions were switched from NC to HC in Group A, from HC to NC in Group B (Fig. 1). The work intensity during training was set to be at lactate threshold (LT) under each NC and HC. During each training session, heart rate (HR) was recorded to monitor the subjects' exercise stress (Polar CIC, Port Washington, NY). Also, plasma lactate ([La]) was analyzed by an enzymatic method (KDK lactate pro analyzer, Japan) during each training. Arterial oxygen saturation was measured by pulseoximetry (MicrO2, Siemens, Germany).
Training protocol. The study consisted of the following phases: The subjects were assigned to two training groups. Group A trained under hypoxic condition (HC; 15% O2) comprised of 2 hours/day, 3 days/week for 3 weeks while Group B trained under the same training protocol under normoxic condition (NC; 21% O2). After each training period, five weeks of self-training under usual field conditions was intervened as washout period. After the self-training, the training condition was switched from HC to NC in Group A, from NC to HC in Group B. The work intensity during training was set to be at lactate threshold (LT) under each NC and HC. The subjects were tested at sea level before and after each training period, and measured O2 uptake (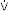 O2), blood samples, and muscle deoxygenation were measured during bicycle exercise test.
O2), blood samples, and muscle deoxygenation were measured during bicycle exercise test.
Hypoxic condition
We simulated HC in the training room by replacing O2 gas in the room air with N2 gas. HT was performed in the room at ambient fractional O2 concentration of 15 % (corresponding to an altitude of approximately 2500 m). O2 concentration in the room was maintained by the system's feed back mechanism which activated a fan that brought in fresh air from the outside whenever the O2 concentration in the room was lower than the O2 set point in the system. CO2 gas in the room was also monitored and the CO2 built up in the room was reduced by using CO2 scrubber. Several air conditioning systems ensured that there were no changes in room temperature and humidity during the training session. The complete set up for simulating normobaric hypoxia in the room was evaluated and approved for use with humans by a Norwegian governmental safety organization.
Experimental protocol
Bicycle exercise test was performed at sea level within one week before and after each training period for evaluating training effects. Subjects had a brachial arterial line and then rested for one hour prior to exercise. After the resting blood samples were taken, subjects moved on to the stationary bike (Lode, Netherlands).
Measurements were carried out on two types of incremental bicycle exercise tests. Before the submaximal test (Submaximal), 50 W warming up was performed for 10–15 min. Submaximal was started just below LT (stage 1), which was determined prior to the test. The workload (~25 W) increased every 5 min to stage 5 for determination of
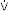
O2 at LT (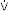 O2@LT). More than 15 min after the end of Submaximal,
O2@LT). More than 15 min after the end of Submaximal, 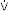 O2max protocol (Vmax) was carried out to determine
O2max protocol (Vmax) was carried out to determine 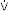 O2max. Vmax test also started just below LT, and ~25 W was added every 30 sec until voluntary exhaustion. After each exercise protocol, subjects continued to cycle at 50 W in order to avoid the health risks due to sudden cessation of exercise.
O2max. Vmax test also started just below LT, and ~25 W was added every 30 sec until voluntary exhaustion. After each exercise protocol, subjects continued to cycle at 50 W in order to avoid the health risks due to sudden cessation of exercise.
Expired gas was collected into Douglas bags. Gas fractions from the Douglas bag were measured by mass spectrometer (Ametec process & analytic instruments Division, USA). [La] was analyzed from arterial blood samples immediately following each exercise stage. A heart rate monitor was fitted around the chest of each subject for the biotelemetry of the heart rate. Arterial oxygen saturation was measured by pulseoximetry. Muscle oxygenation of vastus lateralis (VL) was monitored using NIRcws. The muscle oxygenation was measured after each training period but not before each training period.
Hematology assessments
Plasma volume was measured by using the Evans blue dye indicator-dilution technique. After the subject rested in a supine position for 30 min, a baseline blood sample was drawn and a known quantity of Evans blue dye was injected. Venous blood was drawn 10, 20 and 30 min after injection of the dye. Samples were spun and measurements of absorbance were taken at 620 and 740 nm by spectrophotometry (UV-1202, Shimadzu, Japan). Hematocrit was measured by averaging five runs on a Sysmex K-1000 total blood analyzer (TOA Medical Electronics, Japan). Blood volume was estimated by dividing plasma volume using one minus hematocrit; appropriate corrections were used for trapped plasma and peripheral sampling. Total red cell volume was defined as blood volume minus plasma volume. Hb concentration was measured from a 3 ml sample of venous blood in an EDTA-vacutainer, and it was measured with the same analyzer as hematocrit. Ferritin was measured on plasma samples taken before and after each training period by an immuno turbidimetric method (Modular P, Roche, Swiss).
NIRcws
Oxygenation of the vastus lateralis muscle was measured by NIRcws (HEO-100, OMRON, Japan). The basic principle of this NIRcws device has been discussed in detail in previous studies [9, 11, 13]. The NIRcws probe contains a light source and an optical detector, with a distance of 3.0 cm between the light source and the detector. Thus, the depth of penetration is evaluated to be ~1.5 cm, as extensively discussed previously [14, 15]. A pair of two-wavelength light emitting diodes, with wavelength of 760 and 850 nm, were used as the light source. A silicon photodiode was used as the photodetector. In this study, oxygenated Hb and/or Mb changes (ΔOxy-Hb/Mb) and total Hb changes (ΔTotal-Hb) were calculated using the algorithm reported previously [13]. Also, deoxygenated Hb/Mb (Δ[Hb/Mb]), and muscle tissue deoxygenation (Δ[Deoxy]) were calculated by Δ[Total-Hb] - Δ[Oxy-Hb/Mb] and Δ[Hb/Mb] - Δ[Oxy-Hb/Mb], respectively.
In NIRcws measurements, the absolute O2 concentration or saturation determination is difficult because of unquantifiable biophysical quantities such as optical path length [12]. In muscle oxygenation measurement, a subctaneous fat layer greatly affects the detected light intensity [9]. As the fat layer thickness varies greatly in humans, optical densities of Δ[Total-Hb] and Δ[Oxy-Hb/Mb] cannot be compared between individuals. In this study, therefore, relative deoxygenation change at each stage during Submaximal (%d [Deoxy]) was normalized by the full deoxygenation during Vmax at each test. The %d [Deoxy] was evaluated during the last 30 sec of each stage. The muscle oxygenation recovery was taken from the maximal deoxygenation measured over the last few seconds of exercise and the minimum deoxygenation measured in the overshoot recovery. Half time reoxygenation (T1/2), the time to reach a value of half-maximal recovery, was determined after Submaximal. The probe was firmly attached to the skin overlying the lower one-third of VL muscle (~12 cm from the top of patella). No sliding was observed in any subjects.
Since Mb has similar absorption spectra to Hb, NIRS signal gives mixed information of both Hb and Mb. However, it is reported that Mb concentration is no greater than 25% [11] or 20% [16]. Therefore, we can conclude that the signals are derived mainly from Hb. The specific probe position was recorded as the distance from the top of the patella in the first experiment in December, and the probe was placed at exactly the same location for each test after December.
Statistics
The results are presented as mean value ± standard error (SE). The hematological assessments and measurements parameters during Vmax test were statistically analyzed with one-way ANOVA. The HR, [La] and %d [Deoxy] at Submaximal were compared by two-way ANOVA (time and training). The monitored parameters during each training and T1/2 after Submaximal were compared by paired t-test. Statistical significance was set at p < 0.05 for all comparisons.
Results
Normoxic and hypoxic training
Since relative exercise stress was the same in HT and NT, absolute workload was significantly lower in HT than NT (p < 0.01, Table 1). SaO2 during training was also significantly lower in HT than in NT (p < 0.01, Table 1). However, there were no differences in [La] and mean HR during each training, respectively.
Hematological changes
All hematological assessments were summarized in Table 2. No changes in Hb concentration, blood volume, and plasma volume were observed in subjects before and after each training period. Therefore, Hct and CaO2 were also not significantly increased from the initial level in each training condition. Plasma ferritin concentration, which reflects iron reserves, was not significantly different before and after each training periods. In summary, no difference was observed in any hematological parameter in this study for measurements taken before and after each training period (Table 2).
Exercise test
The HR and [La] during Submaximal are shown in Fig. 2. No significant differences in HR and [La] were observed between Post NT and HT. Neither 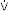 O2max nor peak power at Vmax were significantly improved after each training period (Table 3). Also,
O2max nor peak power at Vmax were significantly improved after each training period (Table 3). Also, 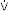 O2@LT was not significantly changed before and after each training. Peak [La] at Vmax was not different either. In summary, no improvement of cardiorespiratory was found post HT and NT.
O2@LT was not significantly changed before and after each training. Peak [La] at Vmax was not different either. In summary, no improvement of cardiorespiratory was found post HT and NT.
Muscle tissue O2dynamics
A typical example of deoxygenation and Δ[Total-Hb] changes is shown in Fig. 3. In warming-up period, Δ[Total-Hb] was increased and Δ[Deoxy] was decreased compared with resting because of increasing muscle perfusion. Immediately after the start of Submaximal, Δ[Deoxy] increased steeply and then continued to increase until the end of this protocol. After the exercise, Δ[Deoxy] showed a rapid recovery, signifying muscle reoxygenation. The kinetics of Δ[Deoxy] in Vmax test was almost the same as that for Submaximal, but Δ[Deoxy] was larger in Vmax than Submaximal. Normalized Δ[Deoxy] during Submaximal (%d [Deoxy]) after each training period is shown in Fig. 2. After both NT and HT, increase in muscle deoxygenation was observed even at the lowest power (Stage 1; NT: 182.1 W, HT: 183.3 W). Thereafter, there was a steady increase of deoxygenation as the exercise intensity increased. Except stage 1, %d [Deoxy] was significantly higher after HT than NT throughout Submaximal test (p < 0.01). T1/2 after Submaximal in NT (18.8 ± 2.7 sec) was significantly slower than that in HT (13.2 ± 2.6 sec) (Figure 4).
Discussion
The major finding of this study was that HT induced more deoxygenation and faster reoxygenation after Submaximal than NT. However, neither blood nor expired gas parameters were changed before and after each training period. These results suggested that three weeks HT may not give an additional performance benefit at sea level for elite competitive cyclists, even though HT may induce some physiological adaptations on muscle tissue level.
Cardiorespiratory system
One of the most important adaptations in HT is considered to be an increase in Hb concentration and Hct, which increases the O2 delivery and improves aerobic capacity [1, 2, 8]. For this endogenous adaptation (i.e. Hb increase), serum ferritin concentration is decreased. However, the ferritin concentration did not decrease after HT (Table 2). Also, Hb concentration and Hct were not increased by HT either. These results suggest that only two hours of hypoxic exposure and/or training per day may not be sufficient to elicit erythropoiesis, thereby producing no improvements of Hb concentration and CaO2. This may be the main reason why the cardiorespiratory system (i.e. maximal O2 uptake) was not improved by HT.
The [La] during Submaximal was not significantly different between each training period even though %d [Deoxy] was significantly higher in HT than NT (Fig. 2). Brooks [19] already stated that [La] is determined by the size of the lactate pool in the blood such as its distribution throughout the body, its oxidation in the muscle, and its conversion to glycogen in the liver and muscle. In other words, [La] is reflected as the balance between blood lactate appearance and blood lactate clearance. Donovan and Brooks [20] found no significant effects of training on blood lactate appearance during exercise, but lower [La] was observed in trained subjects during exercise as a result of enhanced clearance capacity. In this study, training intensity was adjusted at LT level in each NT and HT, and [La] during training was very similar between each training period (Table 1). Therefore, as the training intensity was adjusted by [La], enhanced blood lactate clearance capacity (lactate turnover and oxidation) cannot be attributed to HT.
Effects of hypoxic training on muscle tissue level
In this study, higher muscle deoxygenation during the test and faster T1/2 after the test were found in post-HT than in post-NT (Fig. 4). One possibility may be due to down regulation of skeletal muscle structure and function by reduced absolute training workload in HT. Since relative exercise stress was the same in HT and NT, absolute workload was significantly lower in HT than NT (p < 0.01, Table 1). We expected to see that decreased work intensity might have resulted in the athletes being detrained when they returned to sea level, and thus may have adversely affected performance [1, 2, 8]. Although not significantly different, peak power during Vmax test in post-HT was 13 W less than pre-HT and post-NT (Table 3). If muscle atrophy happened after HT, reoxygenation time would be fast due to increase in capillary density and decrease in diffusion distance. Also, muscle atrophy may be related to relative deoxygenation change (%d [Deoxy]) during exercise. %d [Deoxy] was normalized by the full deoxygenation during Vmax at each test, and Submaximal was set at absolute same work intensity in all tests. Therefore, if peak power were to be slightly decreased, then the same workload would cause more deoxygenation. In other words, %d [Deoxy] during the same workload may be higher in post-HT than in post-NT if muscle atrophy happens.
Another possible explanation, quite different from the one above, may be due to up regulation of skeletal muscle structure and function by HT. In a number of studies an increased capillary network [3, 4, 21] and an elevated oxidative potential of skeletal muscles, including myoglobin content [5], mitochondrial content [3, 21], and oxidative enzyme activities [5], have been reported to accompany hypoxic training. Also, it is well known that endurance training improves muscle O2 extraction during submaximal exercise as demonstrated by a lower venous femoral PO2 [22, 23]. Therefore, it is suggested that O2 extraction may be improved in activating muscle during Submaximal caused by HT. In addition, we observed that T1/2 after Submaximal was significantly faster post-HT than post-NT (p < 0.001, Fig. 4). As we used bicycle exercise, which elicits a greater increase in cardiac output and blood flow to the working muscle, it is speculated that the T1/2 after this type of exercise may more reflect the "washing" of O2 supply than more than O2 consumption [24]. Therefore, faster T1/2 seen after HT implies that O2 supply to the activating muscle may be much higher. Further NIRcws studies are needed to define the conclusions for up or down regulation of muscle function by HT.
Discrepancy between cardiorespiratory system and skeletal muscle after HT
We found that blood and expired gas parameters were not changed by HT even though HT may cause some adaptations on muscle tissue level. Some HT studies have also reported that HT did not improve cardiorespiratory system (maximal O2 uptake) but muscle oxidative capacity, which was determined by phosphocreatine resynthesis rate [25] and mitochondrial volume density and capillary length density [21]. Although we cannot conclude whether or not HT caused up or down regulation on muscle function, three weeks of HT had no effect on the gain of cardiorespiratory system in either case.
Limitations of the study
It is important to note that the sample size (n = 8) was too small to apply our findings to a larger population. Certainly more data should be collected in order to generalize the findings obtained in this study.
Training effects evaluated by NIRcws were compared only for post-training in each subject because baseline data of muscle deoxygenation and reoxygenation were not measured in pre-training periods. However, all subjects trained in both HC and NC. Also, five weeks of self-training under usual field conditions was implemented as a washout period after each training period. Several studies have reported that any improvements due to HT diminish very rapidly upon due to acclimatization at sea level [1, 4, 5]. Furthermore, it is reported that cardiorespiratory and muscle oxidative functions are adapted to variable levels of energy demands within a few weeks [26, 27]. Hence, if the muscle oxidative capacity and capillarization were improved by each training, the improved muscle oxidative function may have been diminished by the time of another baseline test in January.
Conclusions
Three weeks of HT and NT did not improve performance, arterial O2 content, 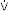 O2@LT or
O2@LT or 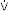 O2max in endurance athletes. In contrast, deoxygenation change during exercise in the vastus lateralis was higher after HT than NT. Furthermore, half time of reoxygenation was faster after HT than NT. These results suggest that three weeks HT may not give an additional performance benefit at sea level for elite competitive cyclists, even though HT may induce some physiological adaptations on muscle tissue level.
O2max in endurance athletes. In contrast, deoxygenation change during exercise in the vastus lateralis was higher after HT than NT. Furthermore, half time of reoxygenation was faster after HT than NT. These results suggest that three weeks HT may not give an additional performance benefit at sea level for elite competitive cyclists, even though HT may induce some physiological adaptations on muscle tissue level.
References
Stray-Gundersen J, Chapman RF, Levine BD: "Living high-training low" altitude training improves sea level performance in male and female elite runners. J Appl Physiol. 2001, 91: 1113-1120.
Levine BD, Stray-Gundersen J: The effects of altitude training are mediated primarily by acclimatization, rather than by hypoxic exercise. Adv Exp Med Biol. 2001, 502: 75-87.
Desplanches D, Hoppeler H, Linossier MT, Denis C, Claassen H, Dormois D, Lacour JR, Geyssant A: Effects of training in normoxia and normobaric hypoxia on human muscle ultrastructure. Pflügers Arch. 1993, 425: 263-267.
Mizuno M, Juel C, Bro-Ramusen T, Schibye B, Rasmussen B, Saltin B: Limb skeletal muscle adaptation in athletes after training at altitude. J Appl Physiol. 1990, 68 (2): 496-502.
Terrados N, Janson E, Sylven C, Kaijser L: Is hypoxia a stimulus for synthesis of oxidative enzyme and myoglobin?. J Appl Physiol. 1990, 68 (8): 2369-2372.
Bailey DM, Davis B, Romer L, Castell L, Newsholme E, Candy G: Implication of moderate altitude training for sea-level endurance in elite distance runners. Eur J Appl Physiol. 1998, 78: 360-368. 10.1007/s004210050432.
Telford AD, Graham KS, Sutton JR, Hahn AG, Campbell DA, Creighton SW, Cunningham RB, Davis PG, Gore CJ, Smith JA: Medium altitude training and sea-level performance. Med Sci Sports Exerc. 1996, 28: S124-
Chapman RF, Stray-Gundersen J, Levine BD: Individual variation in response to altitude training. J Appl Physiol. 1998, 85 (4): 1448-1456.
McCully KK, Hamaoka T: Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle?. Exer and Sport Sci Rev. 2000, 28 (3): 123-127.
Boushel R, Piantadosi CA: Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000, 168 (4): 615-622. 10.1046/j.1365-201x.2000.00713.x.
Chance B, Dait TM, Chang C, Hamaoka T, Hagerman F: Recovery from exercise induced desaturation in the quadriceps muscle of elite competitive rowers. Am J Physiol. 1992, 262: C766-C775.
Hamaoka T, Albani C, Chance B, Iwane H: A new method for the evaluation of muscle aerobic capacity in relation to physical activity measured by near infrared spectroscopy. Med Sport Sci. 1992, 37: 421-429.
Shiga T, Yamamoto K, Tanabe K, Nakase Y, Chance B: Study of an algorithm based on model experiments and diffusion theory for a portable tissue oximeter. J Biomed Opt. 1997, 2 (2): 152-161.
Delpy DT, Cope M, van der Zee P, Arridge S, Wrary S, Wyatt J: Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1998, 33: 1422-1442.
Matsushita K, Homma S, Okada E: Influence of adipose tissue on muscle oxygenation measurement with NIRS instrument. Proc SPIE. 1997, 3194: 159-165.
Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR: Validation of near-infrared spectroscopy in human. J Appl Physiol. 1994, 77: 2740-2747.
Stray-Gundersen J, Mordecai N, Levine BD: O2 transport response to altitude training in runners. Med Sci Sports Exer. 1995, 27: S202-
Stray-Gundersen J, Hochstein A, Levine BD: Effect of 4 weeks altitude exposure and training on red cell mass in trained runners. Med Sci Sports Exer. 1997, 29: S136-
Brooks GA: Current concepts in lactate exchange. Med Sci Sports Exerc. 1991, 23: 895-906.
Donovan CM, Brooks GA: Endurance training affects lactate clearance, not lactate production. Am J Physiol. 1983, 244 (7): E83-92.
Geiser J, Vogt M, Billeter R, Zuleger C, Belforti F, Hoppeler H: Training high-living low: Changes of aerobic performance and muscle structure with training at stimulated altitude. Int J Sports Med. 2001, 22: 579-585. 10.1055/s-2001-18521.
Roca J, Agusti AGN, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD: Effects of training on muscle O2 transport at
 O2max. J Appl Physiol. 1992, 73 (3): 1067-1076.
O2max. J Appl Physiol. 1992, 73 (3): 1067-1076.Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B: Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol (Lond). 1993, 469: 459-478.
McCully KK, lotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Chance B: Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol. 1994, 77 (1): 5-10.
Kuno S, Inaki M, Tanaka K, Itai Y, Asano K: Muscle energetics in short-term training during hypoxia in elite combination skiers. Eur J Appl Physiol. 1994, 69: 301-304.
Mujika I, Padilla S: Cardiorespiratory and metabolic characteristics of detraining in humans. Med Sci Sports Exerc. 2001, 33 (3): 413-421.
Mujika I, Padilla S: Muscular characteristics of detraining in humans. Med Sci Sports Exerc. 2001, 33 (8): 1297-1303.
Acknowledgements
The authors are grateful to the experimental subjects for participating in this study. They also would like to acknowledge Yuanqing Lin for his expert technical assistance and Eric Sell for his invaluable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
RK carried out the muscle oxygenation studies using NIRS and drafted the manuscript. TK participated in the sequence alignment and performed hematologial analysis. SN participated in the design of the study and carried out muscle oxygenation studies using NIRS. GL carried out muscle oxygenation studies using NIRS. Ø M participated in the sequence alignment and performed expired gas analysis. RS participated in the sequence alignment. JI carried out the muscle oxygenation studies using NIRS. BC participated in study design. JS conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Kime, R., Karlsen, T., Nioka, S. et al. Discrepancy between cardiorespiratory system and skeletal muscle in elite cyclists after hypoxic training. Dyn Med 2, 4 (2003). https://doi.org/10.1186/1476-5918-2-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1476-5918-2-4



